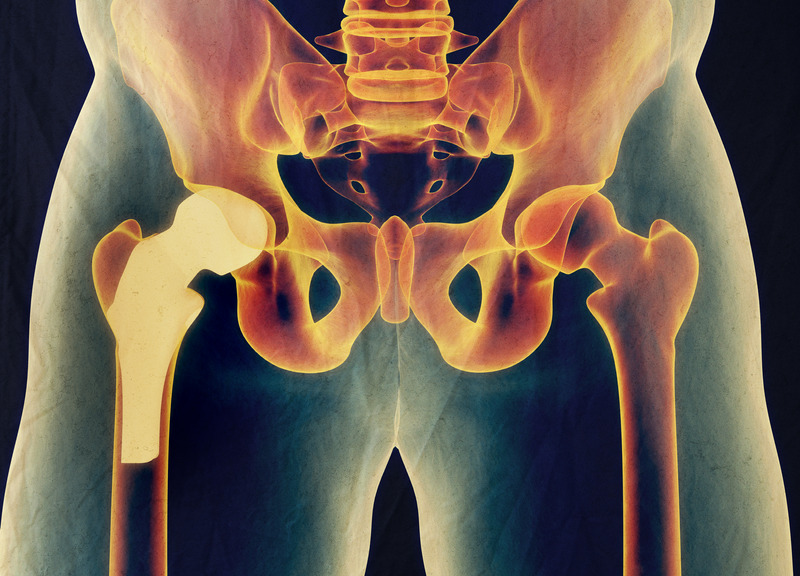
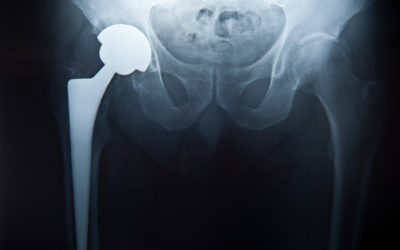
- Metal on metal (MoM) hip resurfacing arthroplasty (HRA) as conceived by Derek McMinn and the late Harlan Amstutz in the 1990’s is unquestionably the proven gold standard for hip reconstruction in 2021.
- HRA is too challenging an operation for most joint replacement surgeons.
- Fear of metallosis is misplaced; but this fear is constantly fanned by most total joint surgeons because their alternative, total hip replacement (THR), is inferior by many measures: worse functional outcome, more residual unexplained pain, worse implant survivorship in young patients, higher rate of debris mediated failure, higher dislocation rate, and higher 10-year all-cause mortality.
- Metallosis with MOM HRA is a solved problem……just place the cup right.
- Trunion corrosion in THR is currently a much greater problem…. And far from being solved.
- There is no cancer risk with MOM bearings, metal allergy is a myth.
- Reversible mild metal toxicity has rarely occurred in past metallosis cases. Severe cardiotoxicity has never been reported in MoM HRA.
- Theoretical concerns with ceramic on ceramic (CoC) HRA are: cracking, squeaking, porous coating debonding.
- Two CoC HRA trials, Imbody H1 and MatOrtho ReCerf are in progress.
- Can a CoC HRA beat the current gold standard uncemented MOM HRA with 99% 15-year implant survivorship in over 5000 cases?
Hip resurfacing is a difficult operation to master and only a few dozen surgeons in the world are proven experts at this operation. Most joint replacement surgeons persist with the standard stemmed total hip replacement (THR) because of technical difficulty of HRA and fear of metallosis, even though THR is clearly inferior to resurfacing. HRA is definitely more technically challenging to perform and few surgeons have the opportunity to get trained. I learned it on my own starting in 1999 and have helped others learn it.
But the fear of metallosis is probably the main reason that most joint replacement surgeons are reticent to learn the operation. Ironically THR now suffer a much higher rate of debris related failures than HRAs do. We now know that metallosis failures in HRA are caused by a combination of acetabular component (cup) design features and the surgeons positioning of this component. My last failure due to metallosis was in a hip implanted in 2009. Since that time, I have implanted over 4000 HRA without a single case of metallosis. In several scientific publications I have first described a “safe zone” for placing the cup called the RAIL (Relative Acetabular Inclination Limit), then developed a simple intraoperative x-ray technique to verify that the component always achieves the RAIL guideline before the operation is completed, and finally verified that metallosis can be prevented in ALL cases in a separate series of over 2000 cases. In summary we have demonstrated that metallosis is a problem of cup malposition and that it is completely preventable. Metal on metal (MoM) bearings are exceedingly safe and reliable as long as they are implanted correctly. Acetabular component malposition with excessive inclination and/or anteversion can lead to edge-loading mechanics and excessive wear resulting in metallosis. Correctly oriented components do not wear excessively; they never develop metallosis.
All artificial joint replacement components are subject to wear and corrosion and all will eventually fail. Failure due to wear or corrosion are only two of many failure modes of hip implants. Metallosis is a condition where there is so much accumulated metal wear debris, from the bearing, in the tissues around the implant that a painful inflammatory condition characterized by large fluid collections and soft tissue swelling occurs. It is NOT an allergic reaction. It is NOT a cancer. It may go by several names such as Pseudotumor (false tumor), ALTR, (adverse local tissue response), ARMD (adverse reaction to metal debris), ALVAL (acute lymphocytic vasculitis associated lesion), or AWRF (adverse wear related failure). Contrary to reports by others, I have found that metallosis rarely causes significant damage to vital structures such as surrounding muscles, nerves, or arteries. Instead, the damage to these structures are usually done by the surgeon revising the failed case when he uses improper technique. These surgeons then claim that the metallosis itself did the damage, rather than their poor technique in the revision surgery. I have revised a few dozen metallosis cases and have published similar revision outcomes with this failure mode as for other causes of HRA or THR failure. In my opinion infection and instability (recurrent dislocation) are far more difficult to solve than metallosis. The problem with metallosis failures is that surgeons first encountering these in the early 2000’s (my first case was 2007) did not know what they were and were understandably inexperienced in dealing with these. Unfortunately, one very prominent university center coined the term “Pseudotumor” and promoted radical excisional surgery. They thought that these were allergic reactions and promoted the idea that every bit of debris had to be removed. This led to operations that damaged muscle and vital structures similar to when cancer is cut out by surgeons. They were wrong. But they had oversized worldwide influence that convinced many surgeons to follow their lead. When a hip with metallosis is opened, a thick white fluid that looks infected pours out. But they are not infected. Then what is left is a thick multilobulated sac with a grey metallic lining and a thick tough wall. If this thick wall and lining is carefully peeled out, no damage is done to the surrounding structures. When this thick wall sits next to a vital structure such as an artery or nerve, some metallosis should be left behind to avoid injury. As long as 90% of the debris is removed and the debris generator (malpositioned cup) is corrected, the problem will resolve. The body will clean up the remaining small amounts of debris. The blood ion levels will fall in half by six weeks and normalize in 1-2 years. Often, I have revised these cases leaving the femoral component and placing a new metal acetabular component in the correct alignment. If you choose to use smaller THR bearings in any revision scenario, the instability rate is high, and if you sacrifice muscle to do a “tumor” operation you make instability worse. Revision hip surgery has worse outcomes and more complications than primary hip surgery, but revision for metallosis in my experience is no worse than revision for any other cause. In fact, failures due to infection and instability (recurrent dislocation) are the most problematic cases by far. In summary, metallosis is a failure mode of MoM HRA. It does not occur if the acetabular component is correctly placed. I had a 1% 10-year failure rate due to metallosis before 2009, and have had none since that time with over 4000 cases. Blood metal ion testing is an excellent screening test. If metallosis does occur, it can be resolved with a revision with a similar outcome to revision for any other failure mode if you choose the right surgeon. It is more important to focus on the overall 10-year failure rate of a certain implant or surgeon rather than focus on the failure rate of metallosis. But proponents of THR understandably want to focus on metallosis because their overall failure rate is much higher.
But THR also have a unique failure mode called trunion corrosion that does not occur in MoM HRA. It is a far greater problem than metallosis in HRA and is far from being solved. The trunion is the connector between the head and the stem. HRA do not have a trunion and therefore cannot exhibit this failure mode. Trunion corrosion can occur in all types of modular metal connections such as the head to stem connection. Modular head/stem connections have given surgeons much more control over fine tuning leg length and tissue tension in a THR and will not be abandoned for one-piece designs. But a rate of failure due to trunion corrosion of 1-5% by 10 years is the cost. It seems that larger heads create more torque on the trunion and are more prone to lead to trunion corrosion. This was the main reason that large bearing MoM THR were abandoned. Therefore, THR have a dilemma: use a larger head and have more trunion corrosion, or use a smaller head and have more instability. Both can be difficult problems to resolve. Trunion corrosion failures have many similarities to metallosis failures due to bearing wear. They also present with a large inflammatory fluid collection with a thick wall. But there is no metallic debris. When the trunion is disconnected, corrosion with black debris is seen on the trunion. Usually most damage is on the head side and the stem trunion is less affected. Usually we just clean up the debris and change the head. But if the stem trunion is too damaged, the stem may need to be removed. This has a high complication rate. There are still many unknowns with this problem of trunionosis. Other than larger head size and modular neck implants, no other design or implant features seem to stand out as a cause. It seems to happen randomly in all THR implants between 5 and 15 years. metal ion testing is a poor indicator of this problem. Metal suppression MRI is the best test. What the long-term success rate is with changing the head is not known. The tissue damage with this problem seems similar or perhaps slightly worse than for metallosis from bearing wear in HRA.
In summary, MoM HRA can fail due to metallosis if the cup is malpositioned. With correct cup positioning in over 4000 cases in the last 12 years we have seen no more cases of metallosis. Instability with HRA which reproduces natural hip mechanics is low. My current metallosis risk is <0.0003%, and instability risk 0.3%. THR can fail due to trunion corrosion at a rate of 1-5% and carries a dislocation risk of 3%. MoM HRA in my hands are far safer than THR.
Other concerns that have been raised with MoM bearing HRA are metal allergy, cancer, metal systemic toxicity, and possible birth defects (if a pregnant woman has one of these implants). The first point to consider is that 99% of total knee replacements have cobalt-chrome femoral components. Many more of these (500,000/year) are implanted in the USA annually, the cobalt ion release is similar to or slightly less than a well-functioning MoM HRA, but none of these issues seem to cause “concern” to joint replacement surgeons who implant them. But they express great “concern” over cobalt release from MoM HRA.
Allergy to metal does exist in the form of nickel skin sensitivity which can be confirmed with a skin patch test. But patients who have this skin sensitivity have been demonstrated to have no higher rate of internal problems with nickel containing joint implants. Both stainless steel and cobalt-chrome alloys contain small amounts of nickel. We have also studied the more sophisticated allergy test named the LTT (lymphocyte transformation test) and found that it also does not predict any clinical problems. With this LTT blood test about 40% of people are diagnosed “allergic” to one or more metals in the implant, but none of these “allergic” patients had clinical problems with this implant. Therefore, we say, the LTT could not be “validated” for its intended purpose. No one else has been able to validate it either, but many internet “experts” recommend it to choose an implant for a specific patient. This is completely unscientific. In fact, metal allergy to an implant is a completely speculative hypothesis with no credible scientific evidence to support it.
The cancer rate is no higher in patients with MoM bearings than with patients with other bearing THR or patients without implants. This has been demonstrated in numerous long-term observational studies. The best is by Visuri from Finland.
Systemic toxicity from cobalt is a real clinical problem A normal Cobalt blood level is under 1.5ug/L. People have died from cardiotoxicity when cobalt sulfate was added to beer in Canada. Blood levels of cobalt were not published. Probably levels around 500ug/L are required. Milder toxicity symptoms such as hearing loss, tinnitus, and neuropathy can occur with levels over 20ug/L. The problem is that all these symptoms commonly occur with aging and other medical conditions such as diabetes and alcoholism. Therefore, it is hard to know when the implant should be blamed. Mom HRA that are edge loading and subsequently lead to metallosis usually have ion levels of around 70ug/L. The lowest level I have ever seen with this problem is 15ug/L. Rarely these patients exhibit signs of mild cobalt toxicity. These symptoms resolve after revision when levels normalize. There has never been a reported case of cardiotoxicity with MOM HRA, only a few cases with failed ceramic on ceramic bearing THR. I recommend removing MoM HRA if the cobalt level is above 20ug/L even if the patient is asymptomatic.
Birth defects have not been reported in babies of women with MoM HRA implants. But there are several reports of women with MoM HRA giving birth to healthy babies. Cobalt levels crossing the placenta are about half of those in moms blood stream. Concern has been raised that this could pose a problem. Birth defects are not uncommon; if the mom has a MOM HRA, would this be blamed as the cause? What if she had a total knee with a cobalt-chrome femoral component? This problem will never be resolved because there are too few women of child-bearing age getting these implants and the number of cases needing to be randomized to perform a properly powered study are too great. And then who knows whether the titanium or ceramic debris coming off THR implants are totally harmless.
Most materials of joint implants, including cobalt and chromium, are already in our bodies naturally and contained in vitamins (B12 cobalamin), and supplements (chromium). Most ions (including sodium and potassium) can lead to problems if their levels get too high, this seems most likely with cobalt. But, I would argue that the overall rate of problems with MoM HRA seems far lower than for THR. This includes severe tissue reactions to debris as well as other failure modes.
Currently, in my hands MoM HRA has a 99% 15-year implant survivorship in all patients, including either sex, any age, any diagnosis, and even with small implant sizes. My rate of failure due to infection is 0/4000. Infection is the single worst complication of joint replacement; far worse than metallosis or trunion corrosion. The benchmark rate in the US is 2.5%. My failure rate due to metallosis is 0/4000. My failure rate due to instability is 1/1000. There is no THR that can come close to these numbers. I would encourage patients to ask about a surgeons infection rate rather than worry about metallosis.
The question is can we do still better with a ceramic on ceramic (CoC) HRA? Ceramic on ceramic bearing uncemented THR are the implants with results that come closest to those of MoM HRA. They are not as functional because they are THR, and their overall failure rate is somewhat higher than my HRA failure rate. They never fail due to metallosis, but they can have trunion corrosion because they are THR. The ceramic can rarely fracture. The implants can sometimes result in loud and unpleasant squeaking. The Stryker CoC THR (the largest selling CoC THR brand) has been recalled in the US for this problem. But, if only bearing wear is considered, CoC are superior, they do have lower wear particle release than MoM. They release zirconia, aluminum, and strontium. It is not known whether these are less harmful to the body than cobalt and chromium. Edge-loading results in squeaking, not catastrophic wear in CoC THR.
What about CoC HRA. The implants need to be very thin walled large bearing sizes. It does look like the newer Biolox forte is strong enough to resist cracking in these configurations. But what if you run on them for 10-20 years? if they crack, ceramic shards in a revision are extremely problematic. What if they squeak loudly when you walk across the room? This seems to be a problem in 1-5% of larger bearing CoC THR. But I suspect it won’t be a problem for HRA because there is no metal stem to resonate. It appears squeaking is caused by an edge-loading bearing, but the vibration is transferred to the titanium THR stem which actually makes the noise. This is the hypothesis…time will tell if CoC HRA will squeak.
What about the implant fixation? This is my primary concern. Will cement bond as well to ceramic as a roughened cobalt-chrome for the femoral component? Probably, but I have already shown that uncemented fixation is superior on the femur. Derek McMinn demonstrated this a long time ago on the socket. For uncemented fixation you need a porous metal layer attached to the component, that allows bone in growth into it. So how well will a titanium porous coating stick to a ceramic implant? This has never been tried before. If these implants come loose there may be a moderate titanium metallosis caused by the failed coating, probably not a big deal. The real question is the durability of this porous coating. If the porous coating debonds from the cup, loosening is the failure mode. My current Biomet HRA has titanium porous coating on a cast cobalt-chrome substrate. In over 5500 case I have never had a femoral coating fail, and only one socket over a 16-year period. On the other hand, Corin MoM HRA that I used previously exhibited a 5% late socket loosening rate due to debonding of the titanium porous coating between 8-15 years. Both companies presumably met the ASTM standard for coating strength. One has a great track record, the other not so much. The current new CoC implants will need to meet the same ASTM standard, but I don’t think we can rely on the ASTM standard. Also, titanium to ceramic has never been tried before. So how long will this last?
There are currently two CoC HRA designs in initial clinical trials in Canada and Europe. Others are being developed. None are available in the US. Justin Cobb in London is leading the Imbody H1 trial and Koen DeSmet in Ghent Belgium and John Antoniou in Montreal are leading the MatOrtho ReCerf trial. Both are made of Biolox ceramic, the H1 is uncemented, the ReCerf has hybrid fixation (cemented femur, uncemented socket).
There is no published short-term data yet. One CoC HRA trial has over 1% failures in the first year. This early failure rate is low, but it is already higher than my 15-year failure rate with uncemented MOM. The other system has a cemented femoral component… I have shown that this results in a 1% 10-year loosening rate not seen if fixation is uncemented. Therefore, it is already doubtful if either of these implants can meet the results of the current gold standard.
When MoM HRA first became available, it made sense for young patients to take a chance on it because THR implant survivorship was so poor (80% 10-year if age < 50). Also, bone is preserved and function is far better for a HRA than a THR. It turns out that the gamble on MoM HRA was a winning bet. Even my earliest results with HRA were more durable for younger patients (10-year implant survivorship of 90%) than for THR. In the last 25 years results have improved to set an extremely high standard which will be hard to equal, and I argue not likely to be exceeded.
I am also involved in CoC HRA development. If these become available in the US I will be involved in early trials. Avoiding the whole tiresome discussion of allergy/toxicity/metallosis would be nice. But if I needed surgery, I would personally choose the proven uncemented MoM HRA which has set the gold standard for hip implant survivorship in the world at 15-year 99% implant survivorship. Hopefully the theoretical pitfalls of ceramic: cracking, squeaking, and debonding of the socket porous coating will not doom the CoC HRA. Do you really want to take a chance, when MoM HRA works so well?